4. Corrosion resistance of magnetic assemblies used in domestic cup yoke-type magnetic attachments
Y. Takada and O. Okuno
Division of Dental Biomaterials, Tohoku University Graduate School of Dentistry
Introduction
Dental magnetic attachments are usually composed of a pair made up of magnetic assemblies and keepers. The attractive force between the magnetic assemblies and the keepers, which are fixed in the denture and root caps, respectively, retains the denture in an oral cavity. A rare earth magnet core (Nd-Fe-B) is covered with magnetic stainless steel, such as SUS444, SUSXM27, or SUS447J1 in domestic cup yoke-type magnetic attachments. These magnetic stainless steels act as yokes for magnetic circuits and protect the magnet core from corrosive environments. In order to prevent closed magnetic circuits, a shield ring made of austenitic stainless steel (SUS316L) is welded in the boundary between the cup and disk yokes with the use of a laser beam. When the dental magnetic attachments work in an oral cavity, the magnetic assemblies, which have such a complex structure, always contact the root caps through the keepers in the corrosive environment. Therefore, keepers and magnetic assemblies are possibly exposed to galvanic corrosion. Since the root caps are generally made of dental precious alloys, it is necessary to examine the corrosion behavior of magnetic assemblies.
Objective
In this study, the corrosion resistance of the magnetic assemblies was electrochemically evaluated by their pitting potentials obtained from the anodic polarization curves on the premise of contact with dental precious alloys.
Materials and methods
 1. Materials
1. Materials
Domestic commercially available cup yoke-type magnetic assemblies, such as Magfit DX800 (Aichi Steel, Japan), GIGAUS D800 (GC, Japan), Hyper Slim, and Hicorex Slim (Hitachi Metals, Japan), were used in this study. The stainless steels used to make the yokes and shield rings and the sizes of the magnetic assemblies are shown in Table 1. The compositions of SUS444 (Nisshin Steel Co., Ltd., Tokyo, Japan), SUSXM27, SUS447J1 (Nippon Koshuha Steel Co., Ltd., Tokyo, Japan), and SUS316L (Nisshin Steel Co., Ltd., Tokyo, Japan) are shown in Table 2. “SUS” indicates the Japanese industrial standards. These stainless steel sheets (10mm x 10mm x 1mm) were also examined as controls. A Type 4 gold alloy (PGA-2, Ishifuku Metal Industry Co., Ltd., Tokyo, Japan) and a silver alloy (Castwell MC, GC Co., Ltd., Tokyo, Japan) were also used to obtain the corrosion potential on the magnetic assemblies when they are in contact with root caps made of precious alloys. The compositions of gold and silver alloys are shown in Table 3.
2. Methods
The anodic polarization curves of the magnetic assemblies and the stainless steel sheets were measured in a 0.9% NaCl solution at 37oC (n=3). The cathodic polarization curves of the gold and silver alloys were also measured under identical conditions. The pitting and corrosion potentials were obtained from the anodic and cathodic polarization curves. The distributions of the elements in the vicinity of the laser welding areas between yokes and shield rings were also analyzed using an X-ray micro-analyzer (XMA) with wavelength-dispersive X-ray spectrometers (WDS) (JXA 8900, JEOL Co., Ltd., Tokyo, Japan) (n=5).
Results
1. Pitting potentials and preferential corrosion areas of the magnetic assemblies
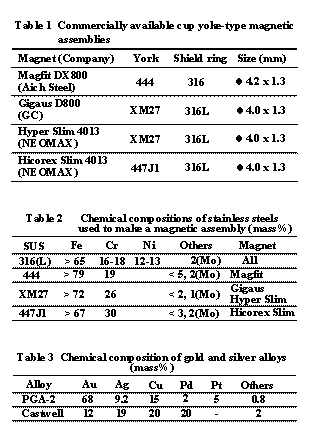
Although the pitting potentials on the magnetic assemblies were expected to be almost equal to those on the SUS316L used to make the shield rings, the anodic polarization curves revealed that all magnetic assemblies tested in this study broke down within the range of 0.75-1.3V, which is significantly higher than that at which SUS316L broke down (Fig. 1). In addition, the magnetic stainless steel for yokes having a higher pitting potential could increase the pitting potential on the magnetic assemblies made of the same stainless steel.
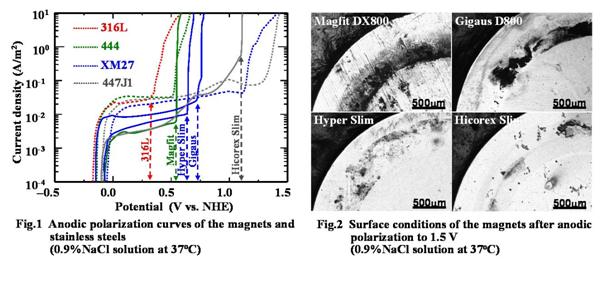 After the magnetic assemblies were subjected to
anodic polarization from the rest potential to 1.5 V, their corrosion areas clearly
revealed that the weld zone on the shield ring of any magnetic assembly had corroded
(Fig. 2). Therefore, the weld zone seemed to be more likely to corrode than
other areas, although it showed a much higher pitting potential than 316L.
After the magnetic assemblies were subjected to
anodic polarization from the rest potential to 1.5 V, their corrosion areas clearly
revealed that the weld zone on the shield ring of any magnetic assembly had corroded
(Fig. 2). Therefore, the weld zone seemed to be more likely to corrode than
other areas, although it showed a much higher pitting potential than 316L.
2. Distributions of elements in the vicinity of the shield ring
The composition images showed that the weld bead deeply covered the surfaces of the shield rings and prevented the exposure of SUS316(L). Since Cr and Ni were found in the entire area of the weld bead and the Cr content was larger than that in SUS316(L), a part of the yokes made of magnetic stainless steels seemed to alloy with the upper side of the shield rings made of SUS316 by laser welding.
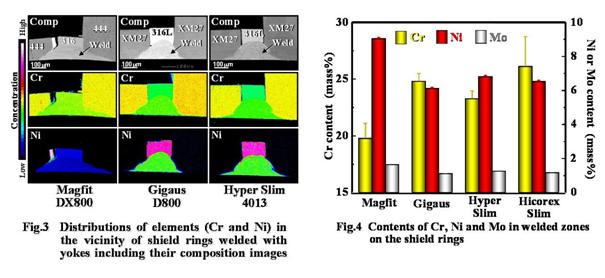
The quantitative analyses using XMA shown in Figs. 3 and 4 indicated that the Cr content in the weld bead was significantly higher than that in 316L (ANOVA, Tukey’s, p<0.05). Therefore, the increase in the Cr content by laser welding possibly contributed to raising the pitting potential and resulted in improving the corrosion resistance of the magnetic assemblies.
Discussion
1. Effect of the Cr content in the yokes on the corrosion resistance of the magnetic assemblies
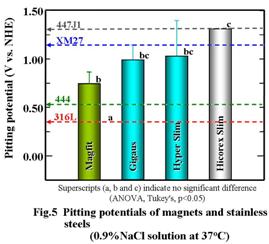 Although pitting corrosion occurs in the
vicinity of the weld zone by electrochemical oxidation, the pitting potentials on
the magnetic assemblies were significantly higher than those of 316L used to make the shield ring or
biomedical implants. The
increase in the Cr content of the yokes raised the pitting potentials of each
magnetic assembly because Cr contained in the yokes was supplied into the weld
bead, which seemed to be more likely to corrode (Fig. 5). “Hicorex slim,”
whose yoke had the largest Cr content, showed the best corrosion resistance of
all other magnetic assemblies tested in this study.
Although pitting corrosion occurs in the
vicinity of the weld zone by electrochemical oxidation, the pitting potentials on
the magnetic assemblies were significantly higher than those of 316L used to make the shield ring or
biomedical implants. The
increase in the Cr content of the yokes raised the pitting potentials of each
magnetic assembly because Cr contained in the yokes was supplied into the weld
bead, which seemed to be more likely to corrode (Fig. 5). “Hicorex slim,”
whose yoke had the largest Cr content, showed the best corrosion resistance of
all other magnetic assemblies tested in this study.
2. Galvanic corrosion of the magnetic assemblies in contact with dental precious alloys
The corrosion potentials and currents of each magnetic stainless steel in contact with the precious alloys (type 4 gold and silver alloys) can be electrochemically obtained from the intersection points of the anodic and cathodic polarization curves (Fig. 6).
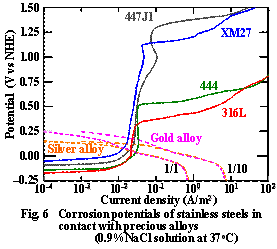 The corrosion potentials on each magnetic
stainless steel were within the passive region in contact with the precious
alloys even when the surface area ratio of (stainless steel)/(precious alloy) was
1/1 or 1/10. In addition, the corrosion potentials were also significantly
lower than the pitting potentials on each magnetic assembly. These
findings indicate that the cup yoke-type magnetic assemblies made of magnetic stainless
steels, such as 444, XM27, and 447J1, show significantly higher pitting
potential than 316L and can maintain passivation and sufficient corrosion
resistance even when they are in contact with dental precious alloys used for
root caps. However, the
shield rings are very narrow in width, about 0.1mm, and their corrosion
resistance is slightly inferior to that of the yoke against the electrochemical
oxidation environment. In galvanic corrosion, therefore, dental alloys
that does not raise the corrosion potential should be selected for use in root
caps.
The corrosion potentials on each magnetic
stainless steel were within the passive region in contact with the precious
alloys even when the surface area ratio of (stainless steel)/(precious alloy) was
1/1 or 1/10. In addition, the corrosion potentials were also significantly
lower than the pitting potentials on each magnetic assembly. These
findings indicate that the cup yoke-type magnetic assemblies made of magnetic stainless
steels, such as 444, XM27, and 447J1, show significantly higher pitting
potential than 316L and can maintain passivation and sufficient corrosion
resistance even when they are in contact with dental precious alloys used for
root caps. However, the
shield rings are very narrow in width, about 0.1mm, and their corrosion
resistance is slightly inferior to that of the yoke against the electrochemical
oxidation environment. In galvanic corrosion, therefore, dental alloys
that does not raise the corrosion potential should be selected for use in root
caps.
Conclusion
The magnetic assemblies tested in this study clearly maintained stable corrosion resistance in an oral cavity because their pitting potentials were much higher than those of 316L for biomedical stainless steel and, furthermore, than the corrosion potentials when they are in contact with precious alloys used for root caps. However, in order to protect the magnetic assemblies from serious galvanic corrosion damage, dental alloys with the lowest corrosion potential possible should be selected for use in root caps.
Acknowledgements
The authors greatly acknowledge Hitachi Metals Co., Ltd., and GC Co., Ltd., for providing the dental magnetic attachments. This study was supported with the NEDO grant (05IS051) and the Grant-in-Aid for Scientific Research (B) of JSPS (18390511).