Effect of Magnetic Fields on Osteoblasts and Fibroblasts in vitro
R.
Fukuzawa1, S. Ozawa1, K. Kubo2, Y. Sugita2,
W. Yoshida2, H. Maeda2 and Y. Tanaka1
1Department of Removable Prosthodontics,
School of Dentistry, Aichi-Gakuin University
2Department of Oral Pathology, School of Dentistry, Aichi-Gakuin University
Introduction
Effects
of magnetic fields on bone biology has been interested, however, those
specificity and mechanism of the magnetic fields on bone have not been revealed
yet1-2). The purpose of this study is to compare osteoblasts
which are responsible to bone formation with fibroblasts which do not make
bone, and explore a mechanism of accelerated bone formation by the magnetic
field exposure.
Materials and Methods
In this study MC3T3-E1 cells that are established osteoblast cell strain form mouse calvaria
and L929 that is standard fibroblast cell strain were used. Those cells were
inoculated onto 12 well plate by 1 x 104 cells per well and
incubated in 37KC, 5%CO2 atmosphere. A time varying electro-magnetic
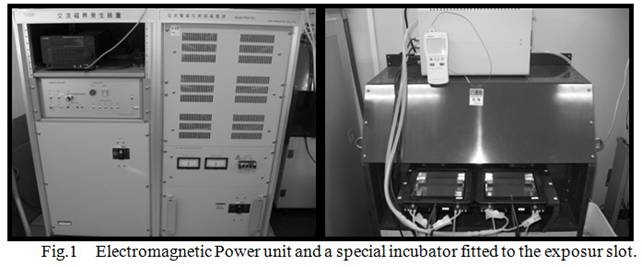
power unit generates maximum 1T magnetic field in the culture area of 120mm x
120mm (Fig.1). Frequency of the magnetic field can also be changed from 0 to
1Hz. This unit equipped cell culture chamber fitted to magnetic field exposure
area. The chamber can carry a culture plate inside the slot. A strength and
frequency of extremely low magnetic fields (ELMF) was set to 0.4T and 0.17 Hz
respectively, and the ELMF was exposed to semi-confluent culture plates for 6
hours.
Cell proliferation was assessed by using a colorimetric
proliferation assay (WST-8 Cell counting kit , Dojindo, Kumamoto, Japan) at day 1, 3, 7, and day 10 after
ELMF exposure. We determined the hormazan content in
the samples by measuring the absorbance at 450 nm. Alkaline phosphatase
(ALP) activities of MC3T3-E1 cells were measured at day 3, 7,and
day 10 after ELMF exposure to evaluate osteoblast
differentiation. ALP activities were standardized by total protein content that
were measured by the Bradford method. Statistical calculation was performed by student
t-test at significant level of 5%.
Results
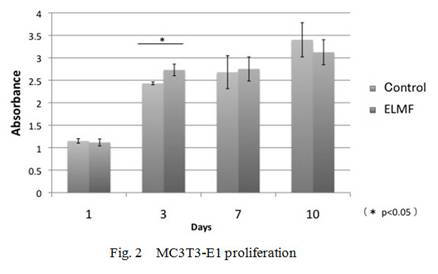
Proliferation of MC3T3-E1 cells were promoted at day 3 after ELMF
exposure, although day 1, day 7 and day 10 cultures did not show significant
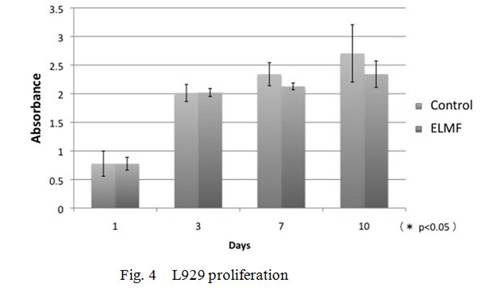
differences between the control and the exposed groups. On the proliferation of
L929 cells, the exposed culture did not show any differences at any time points
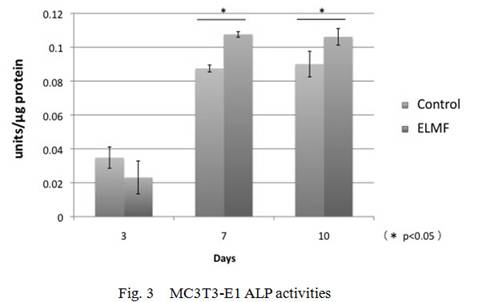
compared with the control cultures. ALP activity in MC3T3-E1 cell significantly
increased at day 7 and day10 as compared with the controls(Fig.2-4).
Discussion
This study revealed that ELMF stimulated proliferation of MC3T3-E1 osteoblast like cells in early stage, and then promoted osteoblastic differentiation at later stage. Whereas ELMF did not have significant effects on proliferation of
L929 fibroblastic cells.
Soda et al. reported that collagen synthesis stimulated by ELMF could
be mediated by p38 MARK pathway and suppressed the collagen synthesis by PI3K
pathway3). Moreover, Nakano suggested that ELMF induced
differentiation of osteoblasts, but not act like Tri-iodothyronine (T3); a regulator of osteoblastic
differentiation4). Since various studies related to the biological
effect of magnetic field have been done, mechanism of bone formation and the
magnetic field stimulation have not been elucidated yet. Further study is
needed to understand ELMF effects on osteoblastic
cell by molecular biological methods.
Conclusion
ELMF of 0.4T and 0.17Hz stimulated mouse osteoblast-like cell proliferation at early stage, and
differentiation to mature osteoblasts, whereas
fibroblasts did not show significant differences in proliferation by the
exposure. These results suggested that osteoblasts have specific response on the magnetic fields.
References
1.
Y. Imaizumi, S. Ozawa , T. Shigemori, et al.:
Effect of extremely low frequency strong magnetic field on proliferation of osteoblastic cells, J J Mag Dent, 15-2, 2006.
2.
K. Shoumura:
Effects of pulsed electromagnetic stimulation for proliferation and
calcification of osteoblast like MC3T3-E1 cell. J Jpn Orthod Soc 56(4) 211-223,
1997.
3.
A. Soda, T. Ikehara,
Y. Kinouchi, et al.: Effect of exposure to an
extremely low frequency-electromagnetic field on the cellular collagen with
respect to signaling pathways in osteoblast-like
cells. The Journal of Medical Investigation, 55, 2008.
4.
Y. Nakano, K. Hosokawa, H. Yamaguchi et al.:
Effects of ELF magnetic fields on physiological functions in cultured osteoblastic cells. IEICE Technical Report
, 101(182), 19-24, 2001.